Optimal Fluid Management
Monty G Mythen
Director Centre for Anaesthesia
University College London
m.mythen@ucl.ac.uk
Introduction
The safe administration of intravenous fluids is one of the most significant advances in the care of surgical patients this century. However, despite advances in the monitoring of cardiovascular variables, the questions of what? when? and how much? remain areas of enormous controversy. The long standing question of what to give, colloid or crystalloid, albumin or synthetic colloid has been the focus of renewed heated debate in recent months. However, “when?” and “how much?” are equally important and hotly debated issues. In our practice at University College London Hospitals we believe that avoiding hypovolaemia is of fundamental importance and that a fluid challenge (most commonly with a colloid guided by oesophageal Doppler monitoring) is the only logical starting point for therapeutic manipulation of the cardiovascular system and optimal fluid therapy.
Diagnosing hypovolaemia
Hypovolaemia can occur as a consequence of a wide variety of pathological processes. The nature of the fluid lost should dictate the choice of replacement fluid. Clinical history, whether first or second hand, in combination with appropriate laboratory investigations, should be the most useful guides to a rational fluid regimen . However, hypovolaemia from whatever cause is an acute medical emergency. Any degree of hypovolaemia jeopardizes oxygen transport and increases the risk of tissue hypoxia and the development of longer term sequelae. The greater the degree and duration of hypovolaemia the greater the risk. Therefore, the initial treatment is to restore the circulating volume as quickly and effectively as possibly and then maintain it.
Hypovolaemia may be divided into 3 catagories. Covert compensated hypovolaemia, overt compensated hypovolaemia and decompensated hypovolaemia.
Covert compensated hypovolaemia
This is the commonest yet least often diagnosed form of hypovolaemia. As a result covert compensated hypovolaemia is probably associated with the greatest morbidity. It refers to the presence of a reduced circulating blood volume associated with no demonstrable physical signs. Price found that healthy volunteers could have 10 to 15% of their blood volume removed with no significant change in heart rate, blood pressure, cardiac output or blood flow to the splanchnic bed (gut etc). However, splanchnic blood volume was reduced by 40%. The subjects in his study had essentially auto-transfused and were maintaining the systemic circulating volume at the expense of the splanchnic circulating volume. More recently Hamilton-Davies et al. performed a similar experiment on healthy volunteers (including myself). We found that approximately 25% of the blood volume could be removed with no effect on the commonly measured cardiovascular variables but, consistent with Price’s findings, the gastric mucosal PCO2 rose immediately followed by a fall in cardiac stroke volume. This same process happens when we donate a unit of blood with no obvious adverse effects. Over the course of the next few hours we feel thirsty and therefore drink more, we also ingest salt and at the same time reduce urine output of salt and water. We make new proteins and blood cells and very soon everything has returned to normal with no sequelae. In hospitalized patients, however, many of the natural compensating mechanisms malfunction and this coupled with the fact that fluid replacement is being determined by a second party, namely the physician, makes hypovolaemia common.
Covert compensated hypovolaemia is extremely difficult to diagnose. In the conscious patient CNS symptoms are the best guide. In the two experiments cited above all of the subjects developed CNS symptoms such as drowsiness, nausea or hiccoughs. Any thirsty patient should be assumed to be hypovolaemic. Urinalysis showing an increased urinary osmolality and decreased sodium concentration is the most useful laboratory investigation.
Although covert compensated hypovolaemia is common and probably contributes significantly to morbidity the majority of patients withstand the insult. If the hypovolaemia persists consequent end organ hypoperfusion may be present for many days before it manifests itself as organ dysfunction. By this time the patient is usually in a state of overt compensated hypovolaemia.
Overt compensated hypovolaemia.
Here there is hypovolaemia to an extent that the reflex mechanisms required to maintain perfusion to vital organs are obvious on clinical examination but the blood pressure is maintained. The patient will demonstrate the manifestations of an increased sympathetic drive with tachycardia, a wide arterial pulse pressure and typically increased systolic blood pressure and cool clammy skin particularly at the hands and feet. There may be other evidence of an inadequate cardiac output such as drowsiness, confusion and an increased respiratory rate.
The majority of laboratory investigations are of little use acutely because of the slow turn around time. Arterial blood gas analysis can be performed rapidly; hypovolaemic patients are commonly hypoxaemic and may have a metabolic acidosis as a consequence of an inadequate cardiac output. Urinalysis, as described above, may support the diagnosis of hypovolaemia but no single test is diagnostic. Determinations of total blood volume are currently impractical.
Except for extreme cases the clinical interpretation of central venous pressure (CVP) by examination of the jugular venous waveform is unreliable and has no place in the management of hypovolaemic patients. If there is any doubt about the diagnosis, particularly in patients with cardiorespiratory disease, the patient needs a CVP catheter. In cases of ventricular dysfunction and / or pulmonary disease there will be a misleading discrepancy between right and left atrial filling pressures. If the information obtained from the CVP catheter is confusing then it is prudent to measure cardiac output. We most commonly do this using the relatively non-invasive Oesophageal Doppler and very rarely by using a pulmonary artery catheter. Whatever methods are employed is important to realise that RA pressure, PAOP, stroke volume and thus cardiac output are all influenced not only by the circulating volume but also by the degree to which the circulation is constricted, the compliance of the right and left heart as well as pain, agitation etc. causing increases in sympathetic tone. Low values are sensitive indicators of hypovolaemia, high values do not necessarily mean that the patient is euvolaemic. Dynamic tests using fluid challenges give much more information and we believe should be the starting point for any therapeutic manipulation of the cardiovascular system. Our usual practice is to give 200 ml of colloid over 5-10 minutes and compare the CVP and Oesophageal Doppler measured stroke volume (not the cardiac output) before the challenge and 5-10 minutes after the infusion has finished. A sustained rise in CVP or PAOP of >3 mmHg and failure of the stroke volume to increase is regarded as the only clinically viable indicator that the intravascular compartment is full.
Decompensated hypovolaemia
This is what many people refer to as shock. The degree of hypovolaemia is such that reflex redistribution of blood flow is insufficient to compensate and vital organs are no longer adequately perfused. If untreated this clinical state rapidly progresses to total circulatory arrest. No special equipment or investigations are needed to make the diagnosis of decompensated hypovolaemia and to start aggressive volume replacement therapy. Inaccurate diagnosis and inappropriate colloid administration is an overrated problem. Delay in the treatment of hypovolaemic shock greatly reduces the chances of successful resuscitation. Most causes of hypovolaemic shock carry a far better prognosis than any condition that presents in a similar fashion but would be made worse by a fluid challenge.
The consequences of hypovolaemia
Decompensated hypovolaemia will result in end organ damage and death if it is not treated rapidly and completely. Probably a far more common and insidious source of morbidity and mortality are the compensated hypovolaemias. As described above a small reduction in circulating blood volume rapidly results in a far more significant reduction in splanchnic blood volume and in particular the supply to the innermost layer of the gut lumen, the mucosa. It is becoming increasingly clear that hypoperfusion of the gut mucosa is of fundamental importance in the pathogenesis of multiple organ dysfunction. Therefore, hypovolaemia is a potential killer in any disease process. The manifestations of persistent covert compensated hypovolaemia may not be seen for many days. Once a patient has overt hypovolaemia the chances of successful treatment are already significantly reduced with the exception of simple acute haemorrhage. Most patients are referred to intensive care units once they have progressed to decompensated shock with established organ failure. By that stage it is probably too late to make a significant difference to outcome. The early recognition and treatment of hypovolaemia is essential in any disease process.
The fluid challenge as a key commponent of optimal fluid management.
We give 200ml blouses of a colloid (e.g. hydroxyethyl starch) as a fluid challenge until there is a sustained rise in CVP by >3mmHg or no increase in stroke volume (figure 2). At oour institutuion we measure cardiac output and stroke volume using an oeasophageal Doppler but any method of measuring stroke volue reliably can be used. The fluid challeng is repeated if the stroke volume falls again or if there any overt signs of end-organ hypoperfusion.
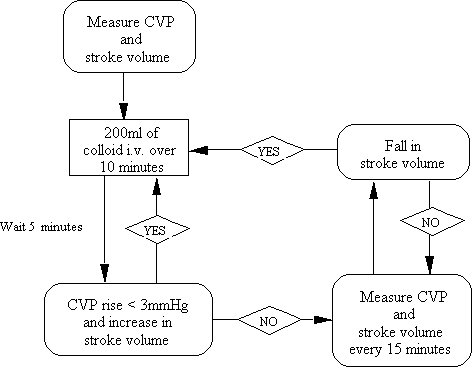
Clinical studies of fluid challenge based treatment regimens
We prospectively studied 60 American Society of Anesthesiology grade III patients with pre-operative left ventricular ejection fraction >50% undergoing elective cardiac surgery. Patients were allocated randomly to a control or protocol group. The control group were treated according to standard practices. After induction of general anesthesia the protocol group received, in addition, 200 ml boluses of a 6% hydroxyethyl starch solution to obtain a maximum stroke volume as outlined above. This procedure was repeated every 15 minutes until the end of surgery except when the patient was on cardiopulmonary by-pass. Patients were followed up post-operatively until discharge from hospital or death. The incidence of gut mucosal hypoperfusion at the end of surgery was reduced in the protocol group (7% vs 56%; p < 0.01) as were the number of patients developing major complications (0 vs 6; p<0.01), days spent in hospital (6.4 (range 5 – 9) vs 10.1 (range 5 –48); p < 0.05) and days spent in ITU (1 (range 1 – 1) vs 1.7 (range 1 – 11); p <0.05).
Remarkably similar results have subsequently been reported in patients undergoing operative repair of fractured neck of femur and non-cardiac major elective surgery randomized to fluid loading guided by Oesophageal Doppler.
Sinclair et al. studied 40 patients undergoing repair of proximal femoral fracture under general anaesthesia. Patients were randomly assigned to receive either conventional intraoperative fluid management or additional repeated colloid fluid challenges guided by the esophageal Doppler monitor to maintain optimal stroke volume. Intraoperative intravascular fluid loading produced significantly greater changes in stroke volume (median 15 ml (95% confidence interval 10 to 21 ml)) and cardiac output (1.2 l/min (0.1 to 2.3 l/min)) than in the conventionally managed group (-5 ml (-10 to 1 ml) and -0.4 l/min (-1.0 to 0.2 l/min)) (P < 0.001 and P < 0.05, respectively). One protocol patient and two control patients died in hospital. In the survivors, postoperative recovery was significantly faster in the protocol patients, with shorter times to being declared medically fit for discharge (median 10 (9 to 15) days v 15 (11 to 40) days, P < 0.05) and a 39% reduction in hospital stay (12 (8 to 13) days v 20 (10 to 61) days, P < 0.05).
More recently, in a prospective, randomized study, Gan et al. assessed the effect of intra-operative fluid optimization guided by esophageal Doppler monitor on length of postoperative hospital stay following major non-cardiac surgery (TJ Gan, Duke University Medical Centre, NC, USA – personal communication). They studied 100 patients who were to undergo major elective surgery with an anticipated blood loss of > 500 ml were enrolled. Patients were randomly assigned to a control group (n=50) that received best standard intra-operative care, or to a protocol group (n=50) that, in addition, received intra-operative plasma volume expansion guided by the Oesophageal Doppler monitor to maintain optimal stroke volume. Groups were similar with respect to demographics, surgical procedures, and baseline haemodynamic variables. The protocol group had a significantly higher stroke volume and cardiac output at the end of surgery compared with the control. Patients in the protocol group had a shorter duration of hospital stay compared with the control group, 5 ± 3 vs. 7 ± 3 days (mean ± SD), with a median of 6 vs. 7 days respectively (p=0.03). These patients also tolerated an oral intake of solid regimen earlier than the control group, 3 ± 0.5 vs. 4.7 ± 0.5 days (mean ± SD), with a median of 3 vs. 5 days, respectively (p=0.01).
Conclusion
Optimal fluid therapy is more about timing and dosage than type of fluid. The Oesophageal Doppler guided fluid challenge can be used effectively and safely to avoid hypovolaemia in surgical patients. The use of the fluid challenge approach to optimize intravascular volume has repeatedly been associated with improved outcome in major surgical patients.
References
Baek SM, Makabali G, Byron-Brown CW, Kusek JM, Shoemaker WC 1975 Plasma expansion in surgical patients with high central venous pressure; the relationship of blood volume to hematocrit, CVP, pulmonary wedge pressure, and cardiorespiratory changes. Surgery 78:304-315.
Gan TJ, Arrowsmith JE, The esophageal Doppler monitor. British Medical Journal; 1997 315: 893-894.
Hamilton-Davies C, Mythen MG, Salmon JB, Jacobson D, Shukla A, Webb AR. Comparison of commonly used clinical indicators of hypovolemia with gastrointestinal tonometry. Intensive Care Medicine 1997; 23: 276-81
Mythen MG, and Webb AR. Intra-operative gut mucosal hypoperfusion is associated with increased post-operative complications and cost. Intensive Care Medicine 1994; 20: 99-104.
Mythen MG, Webb AR. Perioperative plasma volume expansion reduces the incidence of gut mucosal hypoperfusion during cardiac surgery. Archives of Surgery 1995; 130: 423-429.
Price HL, Deutsch S, Marshall BE, Stephen GW, Behar MG, Neufeld GR 1966 Haemodynamic and metabolic effects of haemorhage in man with particular reference to the splanchnic circulation. Circulation Research 18:469-474.
Salmon JB, Mythen MG 1993 Pharmacology and Physiology of Colloids. Blood Reviews 7:114-120.
Sinclair S, James S, and Singer M. Intraoperative volume optimization and length of hospital stay after repair of proximal femoral fracture: randomized controlled trial. British Medical Journal 1997; 315: 909-912.
Singer M, Allen MJ, Webb AR and Bennett ED. Effects of alterations in left ventricular filling, contractility and systemic vascular resistance on the ascending aortic blood flow velocity waveform of normal subjects. Crit. Care Med. 1991;19: 1138-1144.
Singer M, Clarke J, and Bennett ED. Continuous hemodymnamic monitoring by esophageal Doppler. Critical Care Medicine 1995; 17: 447-452.
Weil MH, Shubin H, Rosoff L 1965 Fluid repletion in circulatory shock. Journal Of the American Medical Association 192:668-674.